A Study on Influence of Dilution of Alkaline to Binder Ratios on Fresh and Hardened Behaviour of Geopolymer Mortar


The geopolymer concrete solutions has gained momentum in the recent times, due to environmental concern related to the production of cement in terms of energy consumption and carbon foot print. One such alternative material for the cement is the use of alkali-activated binder using industrial by-products containing silicate materials. The most common industrial by-products used as binder materials are fly ash and blast furnace slag. Slag has been used as cement replacement material due to the latent hydraulic properties, while fly ash has been used as pozzolanic material to enhance physical, chemical and mechanical properties of mortars. The utilization of large proportion of by-products would contribute to the elimination of an environmental problem and to the development of potentially new high-performance material. Recent research has shown that it is possible to use fly ash or slag as a sole binder in mortar by activating them with an alkali component. The activation of material containing mostly silicate and aluminates by a highly alkaline solution will form an inorganic binder through a polymerization process in 1979 [1&3].
The current research is evident that GPM can be cured in sun-dry condition with proportionate combine binder FA&GGBFS [11]. Alkaline to binder ration place significant role in predicting the properties of GPM. To achieve a good consistence in GPM, increasing the alkaline binder ratio, there is significant effect setting of mortar. In this study attempt is made to enhance the consistency of GPM, with dilution of alkaline solution and its impact on Geopolymerization and some basic harden properties are studied.
Material And Methods
Fly ash and GGBS
In the present investigation Class F fly ash and GGBS are considered as binders. The physical characteristics are reported in Table-1.
| Table 1: Physical Properties of Binders | ||
| Particulars | Fly ash | GGBS |
| Residue on 45µ sieve | 24.4% | 2.9% |
| Specific gravity | 2.2 | 2.8 |
| Fineness (Blaine’s air permeability) | 252.30m2/kg | 521.7m2/kg |
Aggregate
Locally available river sand is chosen as filler, which confirm the requirements Indian standards.
Alkaline solution
The locally available sodium silicate and sodium hydroxide solution are used in the present investigation as alkaline solution. The sodium hydroxide is in flakes and pellet with about 98% purity. These pellets were mixed with distilled water to obtain the sodium hydroxide solution of required molarity. In the present study, 4M (4*40g=160g) NaOH solution is considered for investigations. The commercial grade of sodium silicate which has purity of 78% and contains 27% of water is used in the present investigation [7].
Curing of the specimens
The GPM specimens are cured in sun-dry for atmospheric humid factor and varying temperature. The sun-dry curing specimens were covered with a thick polythene sheet in order to reduce the moisture cracks and conventional impression curing is adopted for conventional cement mortar.
Results & Discussion
Influence of addition of water on pH value of combined alkaline solution.
The defining properties for GPM are the alkaline to binder ratio. In the view of enhancing the workability for required strength, an attempt has been made to replace alkaline to binder ratio with water to binder ratio in the mix. The effect of addition of water on the alkalinity of solution is evident thorough pH observation of combined solution of NaOH and Na2Sio3.
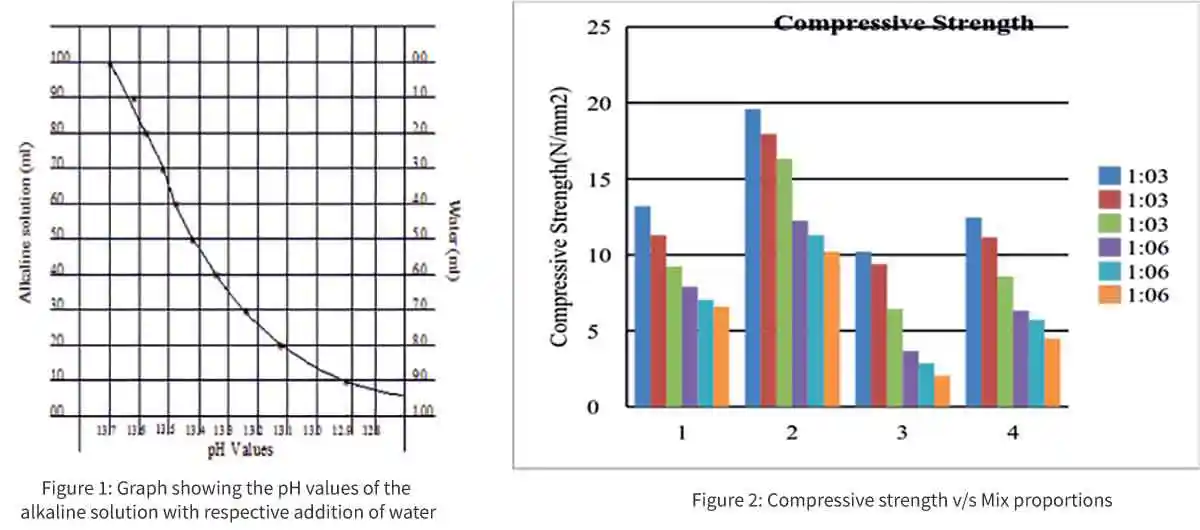
| Table-2: Ph Value of Alkaline Solution with Respective Addition of Water | ||||
| Alkaline solution (ml) | Water (ml) | pH values 1St Day |
pH values 2nd Day |
pH values 3rd Day |
| 100 | 00 | 13.7 | 13.7 | 13.7 |
| 90 | 10 | 13.62 | 13.62 | 13.62 |
| 80 | 20 | 13.58 | 13.58 | 13.58 |
| 70 | 30 | 13.52 | 13.52 | 13.52 |
| 60 | 40 | 13.48 | 13.50 | 13.50 |
| 50 | 50 | 13.42 | 13.42 | 13.44 |
| 40 | 60 | 13.34 | 13.32 | 13.36 |
| 30 | 70 | 13.24 | 13.24 | 13.24 |
| 20 | 80 | 13.12 | 13.14 | 13.14 |
| 10 | 90 | 12.88 | 12.88 | 12.90 |
| 00 | 100 | 7.82 | 7.94 | 7.98 |
The results clearly show that the alkalinity of combined solution does not vary much with the addition of water and there is no significant change in the pH of alkaline solution for the observation of 1, 2 and 3 days in laboratory condition. Hence it can be said that there is no change in the alkalinity of geopolymer solution for continues incremental addition of water.
| Table-3: flow and compressive strength for different mix proportions | |||||||||||
| Cement mortar | Geopolymer mortar | ||||||||||
| Mix No | Mix Proportions | Alkali/Binder Ratio | Flow Values (mm) | Compressive Strength MPa | Mix No | Mix Proportions | W/C Ratio | Flow Values (mm) | Compressive Strength MPa | ||
| 3days | 7days | 3days | 7days | ||||||||
| S1 | 1:3 | 0.64 | 195 | 10.20 | 12.44 | N1 | 1:3 | 0.72 | 195 | 13.2 | 19.59 |
| S2 | 1:3 | 0.68 | 200 | 9.38 | 11.15 | N2 | 1:3 | 0.8 | 200 | 11.28 | 17.95 |
| S3 | 1:3 | 0.72 | 210 | 6.44 | 8.57 | N3 | 1:3 | 0.88 | 210 | 9.24 | 16.32 |
| M4 | 1:6 | 1.13 | 195 | 3.67 | 6.32 | P4 | 1:6 | 1. 3 | 175 | 7.89 | 12.24 |
| M5 | 1:6 | 1.23 | 200 | 2.86 | 5.71 | P5 | 1:6 | 1.39 | 200 | 7.02 | 11.28 |
| M6 | 1:6 | 1.3 | 210 | 2.04 | 4.49 | P6 | 1:6 | 1.5 | 210 | 6.59 | 10.2 |
Influence of dilution of Alkaline to binder ratio on the properties of GPM.
As the geopolymer consists of binders and alkali solution, it is necessary to optimize the solution content required for the matrix. Therefore, the solution content required for the different alkali solution to binder ratios were done for the mixes considered for the study i.e., 1:3 and 1:6. Different ratios have been considered starting from 0.36 to 0.72. The optimum values from the ratios will result in required flow. Primarily few trial mixes were made to fix up the alkali solution to binder ratio using flow table test. Three trials for each mix have been made and tested. The average of all the three trials per each mix has been tabulated in table-4.
| Table-4:Alkaline binder Ratio for Mortar | |||
| Sl No | Mix Proportions | Alkaline/Binder Ratio | Flow Values (mm) |
| 1 | 1:3 | 0.4 | Nil |
| 2 | 1:3 | 0.5 | 157.5 |
| 4 | 1:6 | 0.8 | Nil |
| 5 | 1:6 | 1 | Nil |
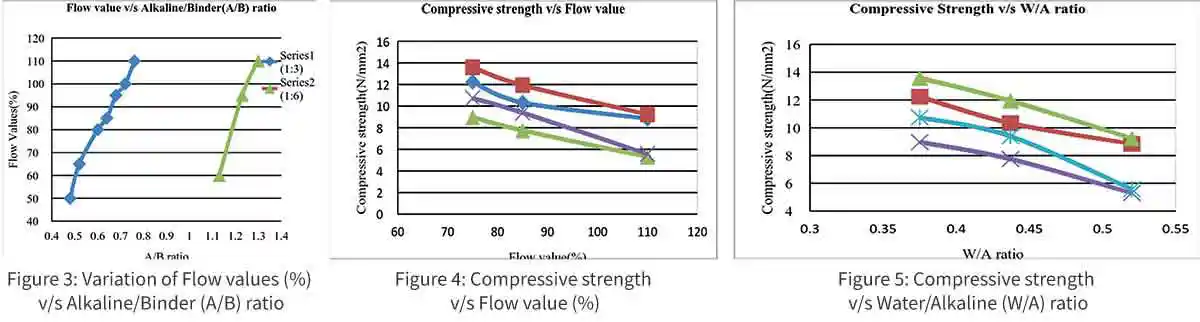
GPM offers good early strength and it is evident that strength of GPM is based on the percentage of alkaline solution, hence, for further work, min alkaline to binder for good flow values are chosen as from TABLE VII and have been kept constant and increasing the amount of water on basis of the flow value or workability in the study. According to IS 2250-1981, the mortar flow should be around 100% to 110% i.e., 200 to 210mm flow depending upon the purpose of mortar.
The trend (fig.3) between the flow values in % and the Alkaline/Binder (A/B) ratio fallows an approximately linear relationship; this relationship is more convenient to use the Alkaline/Binder ratio curve for interpolation.
| Table- 5. Effect of variation of water to alkaline ratio (w/a) for a/b ratio 0.4 of 1:3 mortar/cu-m. | |||||||||||||
| Mix No | Added W/A ratio | Binder (kg) | Alkaline solution (kg) | Sand (kg) | Added water (kg) | Flow table value (%) | Dry Density (kg/m3) | Av. Compressive strength (MPa), for day | |||||
| Fly ash | GGBS | Na2Sio3 | NaoH | Sun-dry curing | Water curing | ||||||||
| 3 | 7 | 3 | 7 | ||||||||||
| M1 | 0.375 | 472.5 | 52.5 | 105 | 105 | 1575 | 78.75 | 75 | 2111.9 | 12.24 | 13.59 | 8.97 | 10.74 |
| M2 | 0.437 | 472.5 | 52.5 | 105 | 105 | 1575 | 91.77 | 85 | 2088.8 | 10.33 | 11.97 | 7.75 | 9.39 |
| M3 | 0.52 | 472.5 | 52.5 | 105 | 105 | 1575 | 109.2 | 110 | 2110.0 | 8.84 | 9.24 | 5.3 | 5.57 |
The strength of mortar primarily depends upon the strength of the binder paste. The strength of paste increases with binder content and reverse with voids and alkaline content. The strength of mortar is only depending upon water/alkaline ratio, provided the mix is workable in GPM. The relation between the water/alkaline ratio and the strength is shown in fig 4 and 5. It is observed that lower water/alkaline ratio achieves higher strength, whereas comparatively higher water/alkaline ratio give lower strength.
Relationship between compressive Strength v/s Flow values
The relation between the strength and the flow values in % is shown in fig 9 and 10. It is evident that increase in the flow value decreases the strength. When the flow value is low, the workability is low, and it gives the higher strength.
| Table-6. Effect of variation of water to alkaline ratio (w/a) for a/b ratio 0.5 of 1:3 mortar. per cu-m | |||||||||||||
| Mix No- S1 | Added W/A ratio | Binder (kg) | Alkaline solution (kg) | Sand (kg) | Added water(kg) | Flow table value (%) | Dry Density (kg/m3) | Avg Compressive strength (MPa) | |||||
| FA | GGBS | Na2Sio3 | NaOH | Sun-dry curing | Water curing | ||||||||
| 3 Days | 7 Days | 3 Days | 7 Days | ||||||||||
| ,M1 | 0.18 | 472.5 | 52.5 | 131.25 | 131.25 | 1575 | 47.25 | 75 | 2120 | 10.61 | 13.05 | 7.35 | 10.34 |
| M2 | 0.2 | 472.5 | 52.5 | 131.25 | 131.25 | 1575 | 52.5 | 85 | 2080 | 15.71 | 12.91 | 8.16 | 7.61 |
| M3 | 0.22 | 472.5 | 52.5 | 131.25 | 131.25 | 1575 | 57.75 | 110 | 2100 | 12.10 | 10.06 | 8.56 | 9.25 |

| Table-7. Effect of variation of water to alkaline ratio (w/a) for a/b ratio 0.8 of 1:6 mortar. | |||||||||||||
| Mix No | Added W/A ratio | Binder (kg) | Alkaline solution (kg) | Sand (kg) | Added water (kg) | Flow table value (%) | Dry Density (kg/m3) | Avg Compressive strength (MPa) | |||||
| FA | GGBS | Na2Sio3 | NaOH | Sun-dry curing | Water curing | ||||||||
| 3 Days | 7 Days | 3 Days | 7 Days | ||||||||||
| S2,N1 | 0.473 | 270 | 30 | 120 | 120 | 1800 | 113.52 | 180 | 2008.5 | 2.85 | 4.11 | 4.76 | 1.27 |
| S2,N2 | 0.509 | 270 | 30 | 120 | 120 | 1800 | 122.16 | 195 | 2048.5 | 2.31 | 3.80 | 4.48 | 1.15 |
| S2,N3 | 0.545 | 270 | 30 | 120 | 120 | 1800 | 130.8 | 210 | 2010.9 | 2.04 | 3.39 | 4.21 | 1.06 |
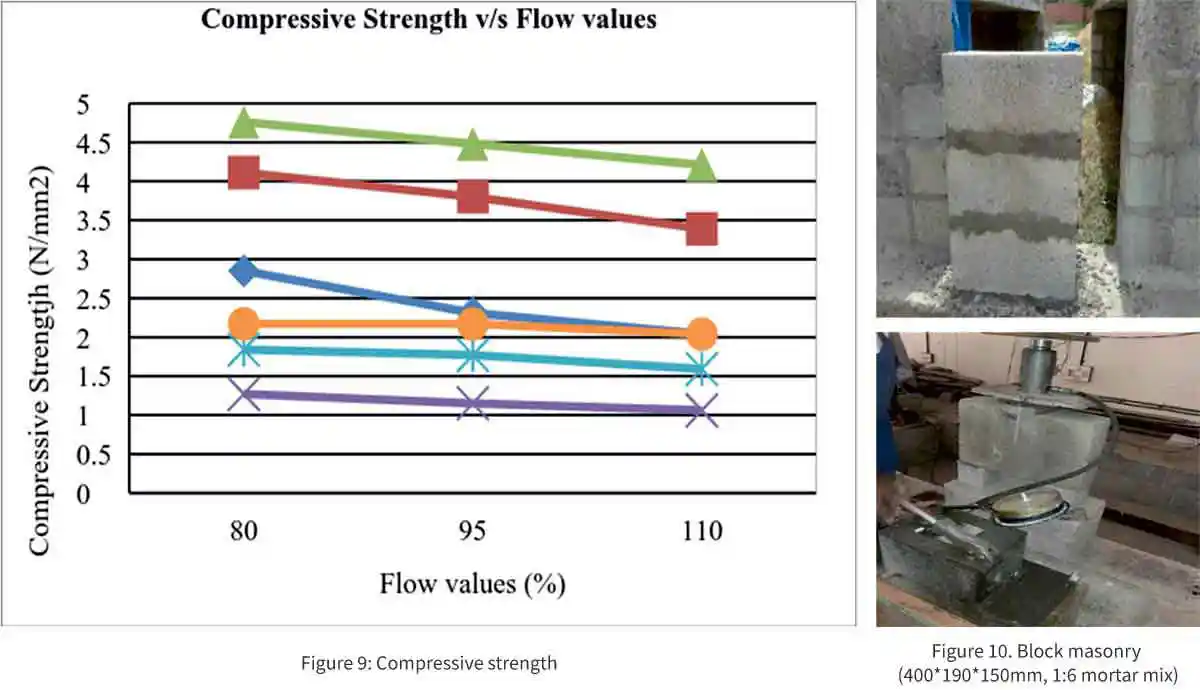
Shear Bond Strength of Geopolymer Mortar
The geopolymer mortar bond strength of masonry specimen is determined by testing masonry triplet under shear. The main objective is to evaluate the strength of mortar mixes with varying proportions by determining the shear strength of triplets and comparing it with the conventional mortar mixes.
| Table-8. Shear strength of solid concrete blocks | ||||
| Sl No | Direction of the plane | Thickness of the mortar joint(mm) | Failure load (N) | Shear strength (N/mm2) |
| 1 | Vertical | 10 | 28000 | 0.52 |
| 2 | Horizontal | 5 | 35000 | 0.31 |
Conclusion
The experimental work on the consistent factors of GPM is evident for the following observations:
- Alkalinity of the solution doesn’t change with dilution with free moisture and may not alter the chemistry of polymerization solution and may not alter the Geo polymer mechanism in GPM.
- Utilization of GGBS as binder to a certain extent (i.e.5-10%) improves the setting time and compressive strength of the geopolymer mortar as compared to conventional mortar. And also added the word self-curing for sundry curing application.
- The results are evident that using fly ash along with GGBS as base material and alkaline solution dilution of free moisture, it is possible to produce mortar of compressive strengths of the order of 5-15MPa. And offers an early strength (about 5-7 MPa in 3 days of sun dry curing) as compared with conventional mortars.
- Bond strength of the GPM (of thickness 5mm and 10mm) is found to be 0.31N/mm2 for horizontal plane and 0.52N/mm2 for vertical plane than the conventional cement mortar.
- Cheng, T. W. and J. P. Chiu. “Fire-resistant Geopolymer Produced by Granulated Blast Furnace Slag.” Minerals Engineering 16(3): 205-210, 2003.
- F.Rendelland R. Jauberthie, “The deterioration of mortar in sulphate environment”. Cement and concrete Research pp. 321-327, 1999.
- J. Davidovits, “Properties of Geopolymer Cements”. Procedings of the first international conference on alkaline cements and concretes. Vol. 1 SRIBM, Kiev, Ukraine, pp. 131-149. 1994.
- Jae Eun Oh, Paulo J.M. Monteiro , Ssang Sun Jun, Sejin Choi , Simon M. Clark, “The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers” Department of Civil and Environmental Engineering, University of California, Berkeley, CA 94720, USA, 2009.
- Jumrat, S. Chatveera, B. and Rattanadecho,P. “Dielectric properties and temperature profile of fly ash-based geopolymer mortar”, International Communications in Heat and Mass Transfer ,2010 Elsevier.
- K.K.Deevasan, (2008) A Study on -“Geopolymer concrete using Industrial effluent”, M.Tech Thesis (Department of Civil Engineering; BMS College of Engineering), Submitted to Visvesvaraya Technological University –Karnataka
- M. D. Cohen and B. Mather, “Sulfate attack on concrete”. Research needs ACI materials Journal, pp. 62-69, 1991.
- Mohammed Saleh. (2007). “A Study on Geopolymer Concrete incorporating Flyash”, M.Tech Thesis (Department Of Civil Engineering; BMS College of Engineering), Submitted to Visvesvaraya Technological University –Karnataka.
- Mohammed,M. Kamarudin,H.I, Niza,K. and Zarina,Y. “Review on fly ash-based geopolymer concrete without Portland Cement”, Journal of Engineering and Technology Research Vol. 3(1), pp. 1-4, January 2011.
- Palomo, M.W. Grutzeck, M.T. Blanco,“Alkaline activated fly ashes cement for the future”. Cement and concrete research, pp. 1323-1329, 29. 1991
- Prashanth V P, R.V. Ranganath and M.S. Sudarshan “Sun cured alkali activated concrete- Some basic properties”, International Conference on “world of concrete India”, October 24-26, Hyderabad – 2013.
- Wallah, S. E., & Rangan, B. V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties, Research Report GC 2. Perth: Faculty of Engineering Curtin University of Technology. 2006.
NBM&CW December 2020


















