Re-alkalization of Carbonated Reinforced Cement Concrete Members

M. Raja Shaker, Research Scholar; Prof. Ramesh R Reddy, Dean and Professor, Department of Civil Engineering, University College of Engineering, Osmania University, Hyderabad.
The process by which Carbon dioxide/Carbon monoxide from the atmosphere penetrates into concrete and reacts with Calcium Hydroxide to form Calcium Carbonates is known as Carbonation. Carbonation results in to shrinkage of concrete. Carbon dioxide in the presence of moisture changes into dilute Carbonic Acid and attacks concrete to reduce alkalinity of concrete. The pH of hardened concrete is alkaline and is generally in the range of 12.6 to 13.5. Carbonation will results in reduction of pH of concrete resulting in rupture of passivating layer around the embedded reinforcement which otherwise protects it from corrosion. Carbonation is one of the main reasons for the corrosion of reinforcement, which is almost uniform unlike chloride-induced corrosion. Reinforcement steel corrosion is faster in the presence of chloride ions than that of carbonation corrosion. Several non-destructive tests were carried out for condition assessment of RCC framed structure, which is situated in an industrial environment. Core samples were extracted for compressive strength, chloride content, and carbonation depth. Ultra sonic pulse velocity (UPV) test, Rebound hammer test, half-cell potential measurements, and resistivity measurements were taken. Test results confirmed corrosion of reinforcement was initiated due to carbonation. The corrective action are initiated to restore the building by re-alkalization of the RCC members using migration corrosion inhibitors, and grouting with re-alkalizing cementitious injection grouts to fill up voids, and giving protective breathable coats to prevent the ingression of carbon dioxide, moisture and other acidic fumes. Re-alkalization had improved the pH of concrete considerably.
Introduction
Generally, concrete is expected to give troublefree service throughout its intended life with or without little maintenance. But these expectations could not sustain in many constructions due to structural deficiency, improper workmanship, unanticipated overloading, premature material deterioration, exposure to different aggressive environments etc., Structures built in highly polluted urban industrial areas, aggressive marine environments, and harmful sub soil waters at costal belts are subjected to rapid deterioration. Degree of harshness of environmental condition to which the reinforced concrete structures are exposed became an important consideration for design, in addition to compressive strength. Carbon dioxide/carbon monoxide present in the atmosphere penetrates into concrete and reacts with calcium hydroxide to form calcium carbonate is called carbonation. The alkalinity of hardened concrete reduces due to carbonation effecting rupture of the passivating layer around the reinforcement leading to initiation of corrosion and shrinkage of concrete. Carbonation is one of the main reasons for the reinforcement corrosion, which will be uniform unlike chloride-induced corrosion. The pH of concrete will reduces from 13 to 9 when all the calcium hydroxide present in concrete is carbonated.
Carbonation of Concrete
The concrete comes into contact with carbonic acid resulting from carbon dioxide in the atmosphere, the ensuing carbonation of the calcium hydroxide in the hydrated cement paste leads to reduction of the alkalinity, to pH as low as 8.5, thereby permitting corrosion of the embedded steel. When all Ca(OH)2 in concrete has become carbonated the pH value of concrete will be as low as 8.3 and at that stage the protective layer gets destroyed and steel is exposed to corrosion.[1] The rate of carbonation in concrete directly depends on the water cement ratio of the concrete, i.e., the higher the ratio the greater is the depth of carbonation in the concrete. In consolidate concrete with reasonable quality without any cracks rate of carbonation is expected to be very low. Cement paste contains 25-50 percentages by weight calcium hydroxide Ca(OH)2), with pH of the fresh cement paste is at least 12.5. The pH of a fully carbonated paste is about 7.The concrete will carbonate if CO2from air or from water enters the concrete according to
CO2+ H2O '! H2CO3
Ca (OH)2+H2CO3'! CaCO3+ 2H2O
Alkali + Acid '! Salt + water
It is clear from the above reaction that the alkalinity of concrete gets reduced. When Ca(OH)2is removed from the paste hydrated Calcium silicate hydrate will liberate CaO, which will also carbonate. The carbonation starts from the surface of concrete and proceeds inwards. The rate of carbonation depends on porosity and moisture content of the concrete. The carbonation process requires the presence of water because CO2dissolves in water forming H2CO3. If the concrete is too dry with relative humidity less than 40%, carbon dioxide cannot dissolve and no carbonation occurs. On the other hand it is too wet with relative humidity more than 90%, carbon dioxide cannot enter the concrete and the concrete will not carbonate. Optimal conditions for carbonation occur at a relative humidity of 50% (range 40-90%). Normal carbonation results in a decrease of the porosity making the carbonated paste stronger. Carbonation is therefore an advantage for non-reinforced concrete but it is a disadvantage in reinforced concrete, as pH of carbonated concrete drops to about 7, a value below the passivation threshold of steel.
 The structure is located in an industrial area where reinforced members are continuously exposed to aggressive environment of carbon dioxide, carbon monoxide etc., The presence of carbon dioxide is about 0.03% in rural areas and in improperly ventilated or industrial atmospheres the percentages goes upto 0.3%. Carbonated concrete will exhibit slightly increase in strength due to hardness with less permeability. The reduction of permeability is attributed to loss of water released due to decomposition of calcium hydroxide. Carbonation is a slow process and takes years to reach the cover depth. In concrete, the presence of abundant amount of calcium hydroxide and relatively small amounts of alkali elements, such as sodium and potassium, gives concrete a very high alkalinity-with pH of 12 to 13. It is well known that at the early age of the concrete, this high alkalinity results in the transformation of a surface layer of the embedded steel to a tightly adhering film, that is comprised of an inner dense spinel phase in epitaxial orientation to the steel substrate and an outer layer of ferric hydroxide. As long as this film is not disturbed, it will keep the steel passive and protected from corrosion.
The structure is located in an industrial area where reinforced members are continuously exposed to aggressive environment of carbon dioxide, carbon monoxide etc., The presence of carbon dioxide is about 0.03% in rural areas and in improperly ventilated or industrial atmospheres the percentages goes upto 0.3%. Carbonated concrete will exhibit slightly increase in strength due to hardness with less permeability. The reduction of permeability is attributed to loss of water released due to decomposition of calcium hydroxide. Carbonation is a slow process and takes years to reach the cover depth. In concrete, the presence of abundant amount of calcium hydroxide and relatively small amounts of alkali elements, such as sodium and potassium, gives concrete a very high alkalinity-with pH of 12 to 13. It is well known that at the early age of the concrete, this high alkalinity results in the transformation of a surface layer of the embedded steel to a tightly adhering film, that is comprised of an inner dense spinel phase in epitaxial orientation to the steel substrate and an outer layer of ferric hydroxide. As long as this film is not disturbed, it will keep the steel passive and protected from corrosion.
Once corrosion sets in on the reinforcing steel bars, it proceeds in electrochemical cells formed on the surface of the metal and the electrolyte or solution surrounding the metal. Each cell consists of a pair of electrodes the anode and the cathode on the surface of the metal and an electrolyte. Basically, on a relatively anodic spot on the metal, the metal undergoes oxidation (ionization), which is accompanied by production of electrons, and dissolution. These electrons move through a return circuit, which is a path in the metal itself to reach a relatively cathodic spot on the metal, are consumed through reactions involving substances found in the electrolyte. In a reinforced concrete, the anode and the cathode are located on the steel bars, which also serve as the return circuits, with the surrounding concrete acting as the electrolyte.
CO2+ H2O '! H2CO3
Ca (OH)2+H2CO3'! CaCO3+ 2H2O
Alkali + Acid '! Salt + water
It is clear from the above reaction that the alkalinity of concrete gets reduced. When Ca(OH)2is removed from the paste hydrated Calcium silicate hydrate will liberate CaO, which will also carbonate. The carbonation starts from the surface of concrete and proceeds inwards. The rate of carbonation depends on porosity and moisture content of the concrete. The carbonation process requires the presence of water because CO2dissolves in water forming H2CO3. If the concrete is too dry with relative humidity less than 40%, carbon dioxide cannot dissolve and no carbonation occurs. On the other hand it is too wet with relative humidity more than 90%, carbon dioxide cannot enter the concrete and the concrete will not carbonate. Optimal conditions for carbonation occur at a relative humidity of 50% (range 40-90%). Normal carbonation results in a decrease of the porosity making the carbonated paste stronger. Carbonation is therefore an advantage for non-reinforced concrete but it is a disadvantage in reinforced concrete, as pH of carbonated concrete drops to about 7, a value below the passivation threshold of steel.

Once corrosion sets in on the reinforcing steel bars, it proceeds in electrochemical cells formed on the surface of the metal and the electrolyte or solution surrounding the metal. Each cell consists of a pair of electrodes the anode and the cathode on the surface of the metal and an electrolyte. Basically, on a relatively anodic spot on the metal, the metal undergoes oxidation (ionization), which is accompanied by production of electrons, and dissolution. These electrons move through a return circuit, which is a path in the metal itself to reach a relatively cathodic spot on the metal, are consumed through reactions involving substances found in the electrolyte. In a reinforced concrete, the anode and the cathode are located on the steel bars, which also serve as the return circuits, with the surrounding concrete acting as the electrolyte.
Condition Assessment and Non-Destructive Evaluation
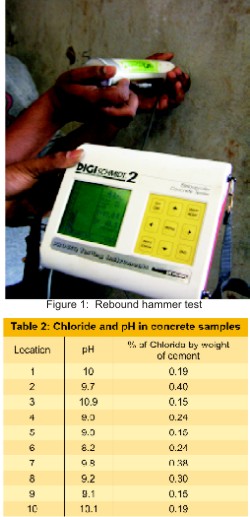
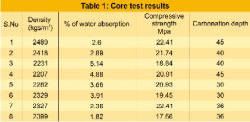
The structure study is located at an industrial atmosphere surrounded by chemical plants. Visual observation was carried out and documented for future reference. The grade of concrete used for the structure is M15. The structure was investigated by conducting non-destructive tests like rebound hammer, core sampling for estimation of carbonation depth, density of concrete, water absorption, and permeability. Rebound hammer test measures surface hardness of the hardened concrete. Low strength concrete having low stiffness will absorb more energy & will give less rebound values. [Figure 1] Powder samples were collected for pH and chloride content. [Table.2] Half-cell measurements and corrosion rate measurements were taken for asserting the presence of corrosion. Ultra sonic pulse velocity test is conducted on selective members to understand the homogeneity, integrity and identification of cracks in the members etc., The average values of UPV are found ranging from 2.4 to 4.1km/see indicating the presence of voids at some locations and also weak bond of the plaster. Core samples were taken with portable core cutting machine. Water absorption, compressive strength and density were determined. The compressive strength of core samples extracted varied from 22 to 26 MPa indicating good strength of core. [Table.1]. The depth of carbonation was checked with 1% phenolphthalein solution. Carbonation depth was found varying from 35 to 60 mm. The change color to pink indicates the portion of unaffected by carbonation. [Figure 2] The carbonation had reached reinforcement at some locations. The pH value of cover zone at many locations were between 9.0 to 10.0 confirming that cover concrete is affected by carbonation, leading to reinforcement corrosion. [3] But the chloride present in concrete is found to be less than 0.4 percentage of weight of cement. This infers that structure is not deteriorated due to presence of chlorides. Measurement of electrochemical parameters was done for assessing and identifying the presence and severity of corrosion. Half-cell potentials with copper-copper sulphate reference electrodes were measured. These test results have indicated the initiation of corrosion process and it is in initial stage. Electrical resistivity measurements were taken. The electrical resistivity is an indication of amount of moisture in the pores, pore system and size of pore. All the non-destructive results conducted confirm that core concrete is having good strength and the cover concrete is carbonated. The presence of chlorides is insignificant. The deterioration of concrete is mainly due to atmospheric pollutants like carbon dioxide and carbon monoxide and also due to aging. Hence structure needs to be protected from CO2,, CO, and chloride attack if any.
Realkalisation of Carbonated Concrete

Realkalisation is an important non-destructive technique used to restore the alkalinity of carbonated concrete for preventing from further corrosion of embedded reinforcement. Removal of carbonated concrete mechanically and replacing the same with new repair mortar involves a lot of work and also consumes time as well as money. Realkalisation helps in increasing the alkalinity of concrete i.e. pH of concrete will be increased to more than 12 initially and keeps the reinforcement in passivating condition for long time. Migration corrosion inhibitors are generally used for realkalisation works. [5]
According to another method of realkalizing concrete, the layers of concrete become carbonated and acidified due to exposure of surface of the concrete. This method assumes and presupposes that the concrete still contains adjacent layers that have not yet become carbonated. A substantially water-tight adherent coating is applied to the surface of the concrete that is exposed to air. Thereafter, the concrete is caused to become saturated with water from a source external to the concrete structure, and this condition of saturation is maintained for a period of time sufficient to effect a diffusion of alkaline materials from the relatively less carbonated layers of the concrete into the relatively more carbonated layers thereof. The carbonated layers thus become realkalized, so that further deterioration of the concrete structure is significantly arrested. [US patented 5049412].
Chloride extraction and realkalisation can be done by electro chemical treatment to halt and prevent corrosion in chloride contaminated and carbonated concrete. [2]. Principle of ion migration is used for removal of chloride ions from the contaminated concrete and simultaneaously pH of the carbonated concrete will be increased through electro-osmosis. Once surface is prepared, a steel or titanium mesh electrode will be attached to surface, and an electrode will be embeded in nontoxic biodegradeble electrolitic media. Once electric field is applied, chloride ions migrate from reinforcement bars inside concrete towards the externally attached electrode. Simultaneously the alkali ions migrate from electrolyte to concrete, thus raising the pH of concrete to original levels.
According to another method of realkalizing concrete, the layers of concrete become carbonated and acidified due to exposure of surface of the concrete. This method assumes and presupposes that the concrete still contains adjacent layers that have not yet become carbonated. A substantially water-tight adherent coating is applied to the surface of the concrete that is exposed to air. Thereafter, the concrete is caused to become saturated with water from a source external to the concrete structure, and this condition of saturation is maintained for a period of time sufficient to effect a diffusion of alkaline materials from the relatively less carbonated layers of the concrete into the relatively more carbonated layers thereof. The carbonated layers thus become realkalized, so that further deterioration of the concrete structure is significantly arrested. [US patented 5049412].
Chloride extraction and realkalisation can be done by electro chemical treatment to halt and prevent corrosion in chloride contaminated and carbonated concrete. [2]. Principle of ion migration is used for removal of chloride ions from the contaminated concrete and simultaneaously pH of the carbonated concrete will be increased through electro-osmosis. Once surface is prepared, a steel or titanium mesh electrode will be attached to surface, and an electrode will be embeded in nontoxic biodegradeble electrolitic media. Once electric field is applied, chloride ions migrate from reinforcement bars inside concrete towards the externally attached electrode. Simultaneously the alkali ions migrate from electrolyte to concrete, thus raising the pH of concrete to original levels.
Repair Methodology Adopted
Concrete penetrating corrosion inhibitors are based on bipolar inhabitation mechanism. These can penetrate even in dense concrete by virtue of its low vapor pressure and natural affinity to metallic surfaces and inhabit corrosion at both anode and cathode surfaces. Conventional inhibitors are based on Calcium nitrate that works only on anodic inhibitors mechanism. Based on the non-destructive tests results it was decided to adopt the realkalisation of concrete for continued use. The existing surface coating of oil bound distemper was removed by scrubbing completely and washed with water and cleaned with compressed air. Holes were drilled at an interval of 500mm center to center along the length of the column on all the exposed sides in such a way that the staggering will be effective to cover maximum area of columns and beams. PVC nozzles are grouted with epoxy grout and air cured. Restoration and increasing the alkalinity concrete, an alkaline inhibitor solution of Calcium hydroxide 2gm/ litre and sodium nitrite of 10 gm/litre solution were grouted in all holes.
After 24 hours, cementious grout of non ferrous fluidiser, non gaseous expanding grout consisting of dry premixed blend of ultra fine special cement, set regulating and reactive chemicals with chlorine free, is mixed with water and injected into concrete at alternate grouting holes. This grouting is required to fill voids and honeycombed locations with cementious grout. It was noted that at some locations considerable quantity of grout was consumed confirming presence of voids. After 24 hours of air cure, single pack ready mix low viscosity epoxy grout was injected. These grouts were injected to take care hairline cracks present in existing concrete surfaces. After the grout, all the PVC nozzles were removed and sealed with epoxy grout.
After 24 hours, cementious grout of non ferrous fluidiser, non gaseous expanding grout consisting of dry premixed blend of ultra fine special cement, set regulating and reactive chemicals with chlorine free, is mixed with water and injected into concrete at alternate grouting holes. This grouting is required to fill voids and honeycombed locations with cementious grout. It was noted that at some locations considerable quantity of grout was consumed confirming presence of voids. After 24 hours of air cure, single pack ready mix low viscosity epoxy grout was injected. These grouts were injected to take care hairline cracks present in existing concrete surfaces. After the grout, all the PVC nozzles were removed and sealed with epoxy grout.
Protective Coatings For Exposed RCC Members
Several methods are available in the market for protecting the exposed surface of concrete. Silanes and siloxanes are used as protective coats in the form of surface treatments. These react with cement matrix to form hydrophobic layer on the walls of the pores within the concrete. Thus protects the concrete from the ingress of water and water born salts. Moisture is required in optimum content for reaction of Silanes and siloxanes with hydrated cement matrix.
Coatings or impregnation on concrete reduce or impede chloride ion penetration, gas penetration to decrease carbonation or water penetration. Coatings consist of continuous film applied on the concrete surface with a thickness in the range of 100 to 300 microns. The coatings in the liquid form are sprayed or brushed on the concrete surface. Different coatings materials are Acrylic, Butadiene, copolymer, chlorinated rubber, epoxy resins, polluter resins, polyurethane, vinyl etc. The other types of materials used for treating the concrete surface are pore liners, which impregnate in the concrete pores and line them with water repellent materials. Materials used in this category as hydrophobic liner are silicons, siloxanes and Silanes. The third category of is pore blockers, which penetrate into the pores and then react with calcium hydroxide and block the pores. The materials available in this category are liquid silicates and silicofluoride.
All exposed RC members were coated with two-component high build epoxy polyamide composition with micaceous iron oxide pigment primer as base. Over this a coat of two-component high build polyamide cured epoxy coating was applied. Inspite of its unchallenged anti corrosion properties, epoxy resins suffer from its inherent limitation like chalking sensitivity in outdoor due to its poor UV stability. It is for this reason all epoxy based high performance coatings are generally over coated with a suitable polyurethane topcoat for colour and gloss retention in exterior exposure. For external surfaces exposed to UV rays, one coat of two-component air drying acrylic polyurethane coating was provided.
Coatings or impregnation on concrete reduce or impede chloride ion penetration, gas penetration to decrease carbonation or water penetration. Coatings consist of continuous film applied on the concrete surface with a thickness in the range of 100 to 300 microns. The coatings in the liquid form are sprayed or brushed on the concrete surface. Different coatings materials are Acrylic, Butadiene, copolymer, chlorinated rubber, epoxy resins, polluter resins, polyurethane, vinyl etc. The other types of materials used for treating the concrete surface are pore liners, which impregnate in the concrete pores and line them with water repellent materials. Materials used in this category as hydrophobic liner are silicons, siloxanes and Silanes. The third category of is pore blockers, which penetrate into the pores and then react with calcium hydroxide and block the pores. The materials available in this category are liquid silicates and silicofluoride.
All exposed RC members were coated with two-component high build epoxy polyamide composition with micaceous iron oxide pigment primer as base. Over this a coat of two-component high build polyamide cured epoxy coating was applied. Inspite of its unchallenged anti corrosion properties, epoxy resins suffer from its inherent limitation like chalking sensitivity in outdoor due to its poor UV stability. It is for this reason all epoxy based high performance coatings are generally over coated with a suitable polyurethane topcoat for colour and gloss retention in exterior exposure. For external surfaces exposed to UV rays, one coat of two-component air drying acrylic polyurethane coating was provided.
Qualification of Repair Methodology
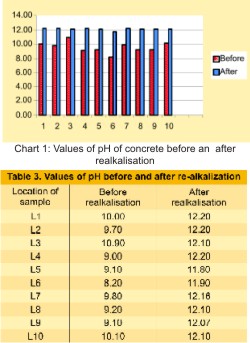
The confirmatory tests were conducted on the repaired structural members. PH test for old concreting before and after injection re-alkalization chemical, etc., were conducted. Powder samples were collected for evaluation of pH of the realkalisation concrete. There is remarkable improvement of pH as given in Table no 3 and shown in Chart 1.
Discussion of Results
Rate of carbonation depends on the level of pore water, concrete grade, permeability of concrete and cover depth. The carbon dioxide diffusion will be very very slow when the concrete pores are filled with water. The already diffused carbon dioxide will result in formation of carbonic acid, which ultimately results in alkalinity reduction. Periodical maintenance like renewal is required for all concrete members of structures exposed to aggressive environment. The design of structures exposed to such aggressive industrial polluted atmosphere condition, appropriate protective coating for reinforcing steel, all the concrete and masonry surfaces shall be specified for execution right in construction stage.
Conclusions
- Alkalinity of concrete can be increased and corrosion of reinforcement due to carbonation can be delayed by using certain corrosion inhibiting chemicals such as nitrites, phosphates, benzonites etc. on hardened concrete.
- Aggressive surrounding environment influence carbonation and corrosion in concrete structures. If the surrounding environment is properly investigated, it is possible to adopt various remedial and precautionary measures so as to delay or minimize carbonation and corrosion initiation in concrete.
- Corrosion inhibitor selected should be capable of penetrating upto steel reinforcement.
- The Corrosion inhibitors used for realkalisation are acting as protective coats for the exposed surfaces of concrete due to property of hydrophobic impregnation
- In the absence of proper investigation on the aggressive effect of the surrounding, the concrete structures have to be built with proper design, construction quality control so as to achieve highly impermeable concrete using mineral admixtures.
References
- V.G.Papadakis et.al Effect of composition and environmental factors and cement–lime mortar coating on Concrete Carbonation _Material and structure 25 no 149 (1992)
- LZA Technol., Sten k Henriksen, David Whitemore, "chloride extraction and realkalisation of Reinforced concrete stop steel corrosion, journal of performance of constructed facilities, volume 12, issue 2, pp 77-84 (May 1998)
- M.Raja Shaker, V.M.Bhat, S.G.Bapat 'Refurbishing of chlorine affected factory building using polymers - a case study" 5thAsian Symposium on Polymers in concrete at Chennai. Organized by Structural Engineering Research Center Chennai Sept 2006.
- M.Raja Shaker, Ramesh. R. Reddy "Sustainability of repair methods adopted for chloride induced distressed rehabilitated work" International Conference on Advances in Concrete and Construction ICACC-2008 organised by Vasavi College of Engineering, Hyderabad in Association with Osmania University, RMIT University, Melbourne, Australia, & South Dakota School of Mines and Technology, USA, 7-9 February 2008
- Hand book on repairs and rehabilitation of RCC buildings published by Director General (Works) CPWD, New Delhi.
- M.Raja Shaker, "Rehabilitation of corroded concrete structures," 14thNational congress on Corrosion Control organized by National corrosion control of India, Karikudi, 18-20, September 2008.
NBM&CW February 2009



















